TTTS. Results
From the viewpoint of pathophysiological mechanisms, our model is clear and simple (Figure 6.3). First, stages I and II TTTS develop if the net fetofetal transfusion increases at a rate in excess of the rate of increasing growth of each twin.
Then, a stuck donor twin and markedly increased colloids in the recipient follow, albeit that recipient hypertension and urine production are not yet excessive. In our model, the strongly increased colloids cause an excess transplacental fluid flow from the maternal to the recipient circulation, a consequence of using Starling’s eqn (10), which mainly exits through the bladder, causing polyhydramnios.
TTTS severity remains limited to stages I or II if the net fetofetal transfusion stabilizes compared with fetal growth, due to the compensating anastomoses that return part of the AVDR transfusion back to the donor. Secondly, TTTS stage IV may develop (Figure 6.4) if the net fetofetal transfusion continues to increase stronger than the rate of increasing fetal growth of each twin. Then, a sequence of events develops that leads to a severely hypotensive donor twin that produces excessive RAS mediators, which are transfused to the recipient by the AVDR anastomosis.
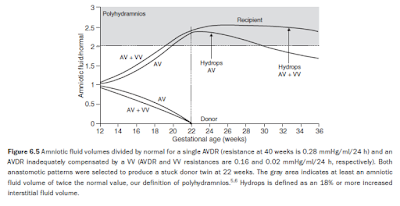
The RAS mediators mitigate the recipient’s polyuria, which causes overfilling, and hence increased arterial and venous pressures (Figure 6.4b), resulting in recipient forward cardiac failure. Subsequently, both the hydrostatic and colloid osmotic pressure gradients between fetal blood and interstitial fluid increase; however, the lymph flow returning from the interstitium into the vascular compartment (not discussed here) is reduced due to the increased venous pressure.6 The overall effect is that the excess transplacental flow now mainly exits into the interstitium rather than the bladder, and onset of hydrops occurs (Figure 6.5).
Interestingly, therefore, increased exit of excess transplacental flow into the interstitial space to cause hydrops necessarily implies a reduced exit into the amniotic cavity, reducing the severity of, or even resolving, the polyhydramnios (see Figure 6.5). (This phenomenon has been described clinically by Trespidi et al in their Figure 1.25) Despite the simplicity of these mechanisms, we acknowledge the increased complexity of this model compared to the previous models,4,5 and refer to our publication for details.6 Our model predicts that recipient blood volumes remain close to their normal values but donor twins become growth retarded, probably a consequence of relating overall blood volumetric growth directly with the net fetofetal transfusion, eqns (13) and (14).
It is important to note that growth discordance may actually not occur in at least 20% of TTTS cases, pointing to a limitation of the model. Furthermore, the model predicts that the amniotic fluid osmolality changes little during pregnancy, even in a severe TTTS case (Table 3 of Umur et al5), in excellent agreement with clinical observations.26 Single and unidirectional AVDR anastomoses, as well as an AVDR inadequately compensated by oppositely directed anastomoses, produce a hydropic recipient twin (see Figure 6.4).
In our model, a hydropic recipient will not spontaneously recover. This is because the donor twin cannot spontaneously recover from its severe hypotension (see Figure 6.4b), so the excess RAS in the recipient, transfused from the donor, sustains, implying the recipient cardiovascular status cannot spontaneously improve either, so hydrops persists (see Figure 6.4c).
However, we acknowledge that this may not actually hold, particularly if there is decompensation or demise of the donor, or thrombosis of the AVDR.27 Simulations of the compensatory capacity of AVRD and AA anastomoses to prevent hydrops in the recipient show that AA is the best in preventing a hydropic recipient twin. However, VV is the best in delaying onset of hydrops following onset of TTTS, owing to the strongly increased venous pressure that always precedes onset of hydrops (see Figure 6.4b).
The fetofetal transfusion along an AVDR anastomosis was found to be as large as 11 ml/ 24 h at 22 weeks, at onset of a stuck donor with 32 ml blood volume (Figure 6.6). This large AV transfusion is possible due to compensation of the corresponding loss of donor fluid by the (maternofetal) transplacental fluid flow, as well as possible transfusion from the interstitial into the vascular compartment. Interestingly, if an AVRD anastomosis is also present, compensating part of the donor’s loss of red blood cells by the AVDR, the net fetofetal transfusion (donor to recipient) even increases to 14.7 ml/24 h at 22 weeks at 34 ml donor blood volume. In other words, our third model simulates a net fetofetal transfusion that can develop without the donor’s short-term demise, predicting a turnover of donor blood volume every 3 days! Interestingly,
AVDR transfusions produced by our model are in unexpected agreement with the experimental value of 27.9 ml/24 hours (see Figure 6.6) that we found recently in five unidirectional AVDRs. These anastomoses remained patent following incomplete TTTS laser therapy, and the new donor required an intrauterine blood transfusion 48 hours before emergency delivery at 30 weeks of gestation.19
DISCUSSION
The development of realistic TTTS mathematical models is challenging. The complexity of fetal physiology may at first make modeling seem a hopeless enterprise: not only is there a paucity of information available on normal fetoplacental cardiovascular function and amniotic fluid homeostasis but also the influence of TTTS on such developments is poorly understood. Therefore, simplified and sometimes empirical descriptions of fetoplacental and amniotic fluid development are unavoidable. In view of the complexity of TTTS pathophysiology, a sequence of models of increasing sophistication, with model testing at each state of development, represents the optimal practical approach.
As a result, we believe that our modeling has provided important principles and trends to illustrate realistic clinical scenarios. In the time period between the first and final draft of this chapter we finalized the description of pulsating arterial flow propagation along the fetal arterial tree.28 We combined this model with our TTTS model,6 extended with the dynamics of blood hematocrit and arterial wall collagen– elastin concentrations (stiffness) and thickness, and described stage III TTTS in our fourthgeneration model.29 Furthermore, very recently, Wee et al30 reported that diameter of draining veins of AV anastomoses increase linearly with gestational age, showing our assumption of AV anastomotic development also to be true. In conclusion, mathematical modeling of TTTS pathophysiology has contributed significantly to understanding the sequence of events that govern the numerous TTTS clinical presentations as well as the efficacy of therapeutic strategies (not shown in this chapter).
SUMMARY OF MODEL ASSUMPTIONS AND PREDICTIONS
• TTTS etiology is that anastomoses develop linearly in length and diameter, proportional to normal placental volumetric development, vs normal growth of the twins.
• TTTS pathophysiology is explained by AVDR fetofetal transfusion, which increases at a rate in excess of the rate of increasing fetal blood volumetric growth of each twin.
• Net fetofetal transfusion can be as high as 33% of the donor blood volume per 24 hours.
• TTTS is caused by one or more (unidirectional) AVDR anastomoses.
• AVDR flow in our model is in agreement with measured AVDR flow, about tens of ml per 24 hours. • The hemodynamic competition between the AVDR capacity (length and diameter) vs the combined capacity of all compensating anastomoses (AVRD, AA, VV) determines whether or not TTTS develops, and TTTS severity. This explains why some but not all monochorionic placentas with anastomoses develop TTTS.
• The probability that an AVRD adequately reduces the flow of the primary AVDR is much smaller than the probability that an AA of equal diameter of the feeding and draining vessels reduces the AVDR flow adequately. This refers to onset of TTTS as well as to onset of a hydropic recipient twin. • A VV anastomosis produces the largest interval between onset of a stuck donor and onset of recipient hydrops.
• TTTS severity is only weakly correlated with an earlier gestational age at TTTS onset (not discussed in this chapter).
• A hydropic recipient will not recover spontaneously.
• A recipient twin that develops hydrops simultaneously reduces its polyhydramnios.





No comments:
Post a Comment