Selective laser photocoagulation of communicating vessels
There are three steps in performing selective laser photocoagulation of communicating vessels (SLPCV). The first step is a diagnostic one, which consists of the identification of all of the anastomoses, differentiating them from individually perfused areas of the placenta (diagnostic fetoscopy step).
The second step consists in the actual lasering of the anastomoses. The third step involves reviewing all of the lasered anastomoses and relasering if necessary, as well as endoscopic review of any other important aspects of the amniotic cavity or fetuses.
Step 1: diagnostic fetoscopy
A diagnostic endoscope is inserted into the amniotic cavity through the trocar sheath and directed towards the placenta. Arteries are identified endoscopically as they cross over veins, and may also have a darker hue. By definition, the vascular equator lies at the terminal end of the communicating vessels. A systematic documentation of all vascular anastomoses is done first.
Deep vascular anastomoses are easily identified by noting that the perfusing artery and vein belong to different fetuses. In some cases, it may be necessary to follow the artery or the vein back to the umbilical cord of the corresponding twin to confirm the alternate origin of the vessels. As mentioned in Chapter 5, deep communications may involve a single artery and vein from each twin (simple AV anastomosis), or a three-vessel or four-vessel cotyledon (Figure 9.10 a–c). In a three-vessel cotyledon, one twin has an artery and vein perfusing the cotyledon, while a third vessel, either an artery or vein from the other twin, is also involved.
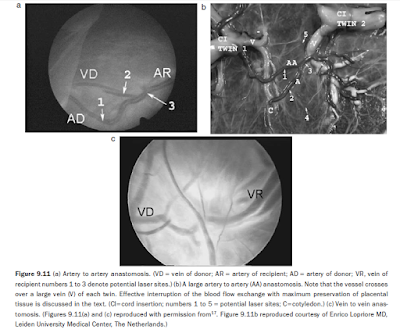
In a four-vessel cotyledon, both fetuses perfuse a common cotyledon with an artery and a vein of each. Deep anastomoses are classified as AVDR if the artery corresponds to the donor twin, or AVRD if the artery belongs to the recipient twin. The size of the anastomoses (‘hair’, small, medium, large, or very large) is also noted. Superficial anastomoses are identified as being arterioarterial (AA) or venovenous (VV) (Figures 9.11a,b), and can be easily seen running uninterrupted between the two fetal circulations. AA anastomoses are more easily detected, as they cross over veins. Superficial anastomoses may be branched or unbranched.16 Typically, the direction of flow in VV anastomoses cannot be determined endoscopically.
AA anastomoses may behave as functional AVDRs or AVRDs, depending on where blood drains (to a vein of the donor or to a vein of the recipient twin, respectively), but can only be seen endoscopically if a significant color difference exists between the two arterial circulations. Once all the anastomoses are documented, the diagnostic endoscope is removed and exchanged for the operating endoscope. In cases where surgery needs to proceed expeditiously, the diagnostic step is undertaken with the operating endoscope and the anastomoses are lasered as they are found.
Step 2: laser photocoagulation of the communicating vessels
The operating endoscope is loaded with the YAG laser fiber. The fiber is kept inside the operating channel to prevent fetal or placental injury upon insertion of the operating endoscope into the amniotic cavity. The YAG laser machine is typically set at 15–20 watts. For large vessels, 25–30 watts may be necessary. The endoscope is placed over the target vessel, and the laser fiber is advanced beyond the tip of the endoscope, enough to see the junction of the laser fiber tip and the fiber.
The site of photocoagulation is chosen, close to the terminal end of the vessel. The vessel is then photocoagulated by applying the energy on to the center of the width of the vessel. If the vessel is deemed large or very large, aiming the laser energy at the center may not be advisable, as it may result in rupture of the vessel and bleeding. Instead, the laser energy is directed to the edges of the vessel (‘inching technique’), until the whole width of the vessel is obliterated (Figure 9.12). Lasering should be continued until the vessel is completely blanched out.
Because the laser is used in continuous mode, the surgeon must decide when to stop firing to avoid perforation of the vessel. Since the only way to gauge effect is through endoscopy, a practical way to avoid vessel rupture is to stop coagulating once a blanching effect is seen. We refer to this technical tip as ‘effect-stop’. The vessel is reassessed, and the photocoagulation process continued until the entire vessel diameter has been obliterated. The process is repeated for each anastomosis, until all the communications have been interrupted.
The sequence in which the vessels are lasered depends on how smoothly surgery has proceeded until this point. If bleeding from the anterior uterine wall has not occurred, particularly with movement of the endoscope within the cavity, the anastomoses may be lasered in the same sequence as they were identified. If bleeding is associated with movements of the endoscope, the anastomoses are lasered in reverse sequence as they were identified, thus moving the endoscope the least. If bleeding occurs as soon as the trocar has been inserted and continues despite corrective maneuvers, the anastomoses are lasered as they are identified without prior diagnostic fetoscopy.


No comments:
Post a Comment